IEC 60601-1 Clause 14 and IEC 62304
IEC 60601-1 Clause 14 and IEC 62304
The software development processes defined in IEC 62304 and IEC 60601-1 Clause 14 follow the V-Model of software development, as illustrated in Figure 6.
In IEC 60601-1 Clause 14, the software system is defined as a sub-requirement under the Programmable Electrical Medical System (PEMS) framework.
During PEMS development, the IEC 62304 standard is applied at the PEMS component level — from defining software requirements, through the assembly of software items, to integration into the overall software system.
This software system then becomes part of the Programmable Electrical Subsystem (PESS).
Therefore, IEC 62304 mainly addresses software verification within the PESS but does not cover full software validation at the system level.
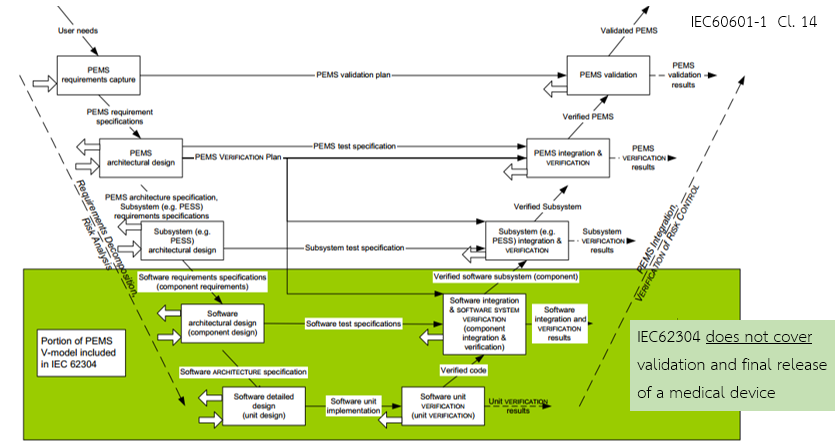
Figure 6 – Software as Part of the V-Model
(Source: ANSI/AAMI/IEC 62304:2006, Medical Device Software—Software Life Cycle Processes)
In Figure 6:
- Green area: Software development process under IEC 62304
- White + Green area: PEMS development process under IEC 60601-1 Clause 14
- Rectangles: Development activities
- Solid arrows: Deliverables (inputs/outputs of each activity)
- Dashed arrows: Deliverables incorporated into the Risk Management File
- Thick arrows (right-facing): Outputs from the software problem resolution process
- Thick arrows (left-facing): Inputs to the software problem resolution process
IEC 62304: Key Clauses
IEC 62304 comprises six major clauses (4–9), as shown in Figure 7 and detailed below.
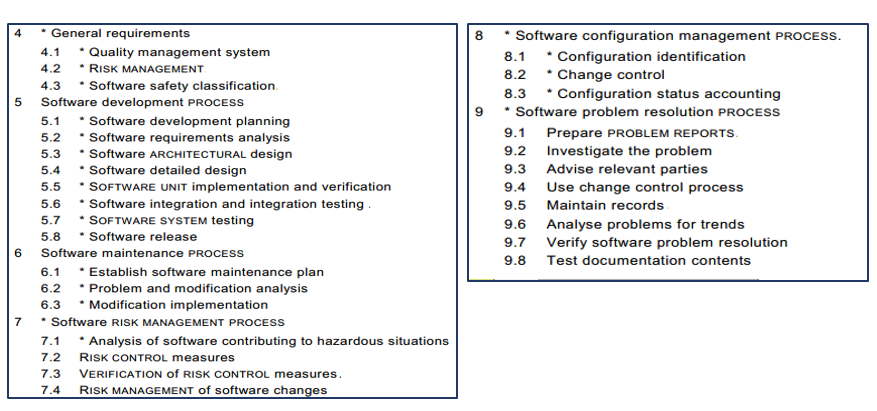
Figure 7 – IEC 62304 Main Clauses
(Source: ANSI/AAMI/IEC 62304:2006, Medical Device Software—Software Life Cycle Processes)
Clause 4: General Requirements
This clause includes several sub-clauses:
- Clause 4.1 – Quality Management System
Requires software development to be conducted under a formal quality management system, generally aligned with ISO 13485. - Clause 4.2 – Risk Management System
Requires a risk management process for software development, referencing ISO 14971. - Clause 4.3 – Software Safety Classification
Defines the classification of software based on safety severity levels:
Class | Description |
A | Software that cannot cause injury or health damage. |
B | Software that may cause non-serious injury. |
C | Software that may cause serious injury or death. |
- Definitions:
- Software System: A complete software entity (or PESS) consisting of multiple software items.
- Software Item: A discrete unit of software, which may itself contain sub-items.
- SOUP (Software of Unknown Provenance): Software with unknown development and safety characteristics.
- Legacy Software: Software with known development and safety characteristics, reused in new systems.
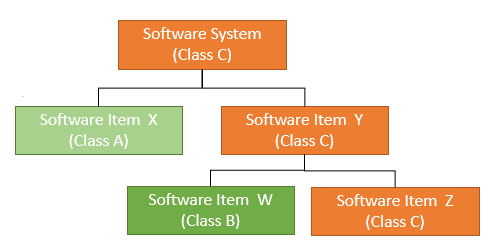
Figure 8 – Software System Structure and Safety Classification
To determine a software system’s safety class:
- Identify all software components within the system.
- Assess the severity level of each component.
- Assign the overall system classification based on the most severe component.
Example:
- If components include Classes C, B, and A → the system is Class C.
- If components include Classes B and A → the system is Class B.
- If all components are Class A → the system is Class A.
For example (see Figure 8):
A software system includes components X and Y, where Y consists of subcomponents W (Class B) and Z (Class C).
→ Component Y is Class C.
→ Even though X is Class A, the overall system becomes Class C due to the most severe classification.
Clause 5: Software Development Process
As shown in Figure 6, this clause describes the step-by-step software development process with the following sub-clauses:
- Clause 5.1 – Software Development Planning
Planning the entire software development lifecycle. - Clause 5.2 – Software Requirements Analysis
Defining and analyzing functional and non-functional requirements. - Clause 5.3 – Software Architectural Design
Designing software architecture, identifying software items, SOUP, and legacy software (see Figure 8). - Clause 5.4 – Software Detailed Design
Detailed design of software units. - Clause 5.5 – Software Unit Implementation and Verification
Coding each software item and performing unit-level verification. - Clause 5.6 – Software Integration and Integration Testing
Combining software items and testing their integration (see Figure 9). - Clause 5.7 – Software System Testing
Testing the complete integrated system (Figure 9 – circular area). - Clause 5.8 – Software Release
Preparing, installing, and verifying the software for deployment.
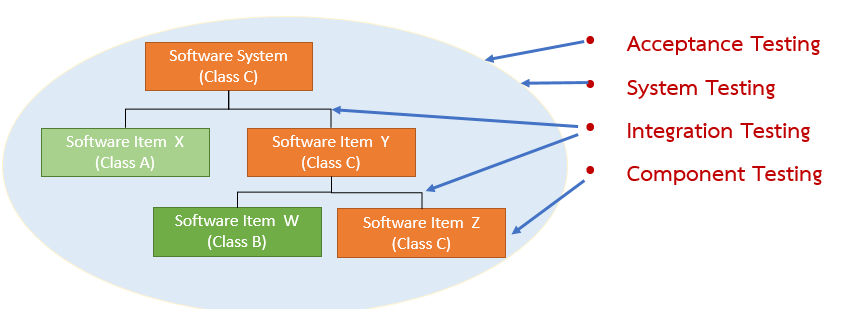
Figure 9 – Levels of Software Testing
Clause 6: Software Maintenance Process
This clause includes:
- 6.1 – Establish Software Maintenance Plan
Define the post-release maintenance plan. - 6.2 – Problem and Modification Analysis
Analyze issues and modifications after system delivery. - 6.3 – Modification Implementation
Implement and verify modifications following analysis.
Clause 7: Software Risk Management Process
Includes:
- 7.1 – Analysis of Software Contributing to Hazardous Situations
Analyze hazards from software items, SOUP, and legacy software. - 7.2 – Risk Control Measures
Define and apply mitigation measures. - 7.3 – Verification of Risk Control Measures
Verify risk reduction effectiveness. - 7.4 – Risk Management of Software Changes
Manage risks arising from software changes.
Note: Clause 7 builds upon Clause 4.2 (Risk Management).
Clause 8: Software Configuration Management Process
Key sub-clauses:
- 8.1 – Configuration Identification
Identify work products and outputs to be controlled (see Figure 10).
Examples include design documents, configuration items (CI), and file naming conventions, e.g.:- F-001-SoftwareDevelopmentPlan → Software Development Plan Form
- File name: xxx-SoftwareDevelopmentPlan
Control also includes:
- File storage (electronic folders or physical cabinets)
- Versioning (see Figure 11 – left)
- Development path definition (see Figure 11 – right)
- 8.2 – Change Control
Manage software changes systematically (see Figure 12). - 8.3 – Configuration Status Accounting
Track status of configuration items — draft, reviewed, approved, etc.
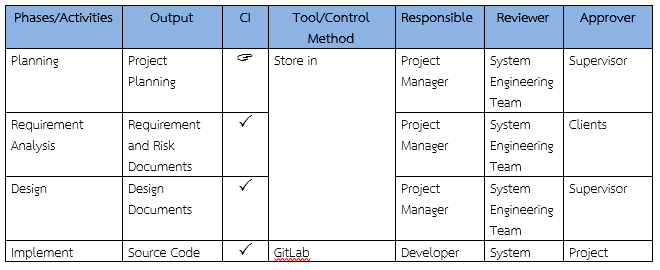
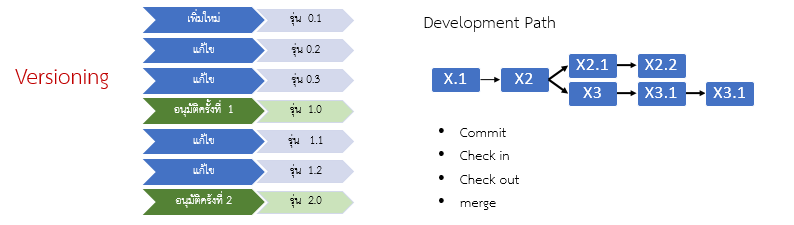
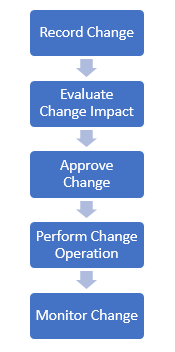
Figures 10–12: Examples of Configuration and Change Management
- Figure 10 – Example Configuration Item
- Figure 11 – Versioning and Development Path
- Figure 12 – Software Change Process
Clause 9: Software Problem Resolution Process
Includes:
- 9.1 Prepare problem reports
- 9.2 Investigate problems
- 9.3 Advise relevant parties
- 9.4 Use change control process
- 9.5 Maintain records
- 9.6 Analyze problems for trends
- 9.7 Verify software problem resolution
- 9.8 Test documentation contents
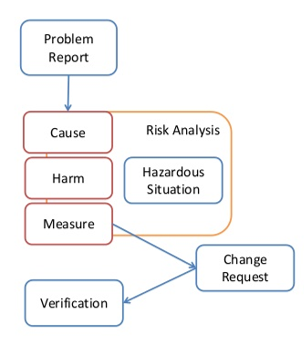
An example process is shown in Figure 13 – Software Problem Resolution Process.
After Software Development
Once medical device software development is complete, manufacturers should verify all required evidence items (see Figure 14).
This Evidence List corresponds to the primary and sub-requirements of IEC 62304, and will be submitted to the Testing Laboratory for certification or regulatory approval.
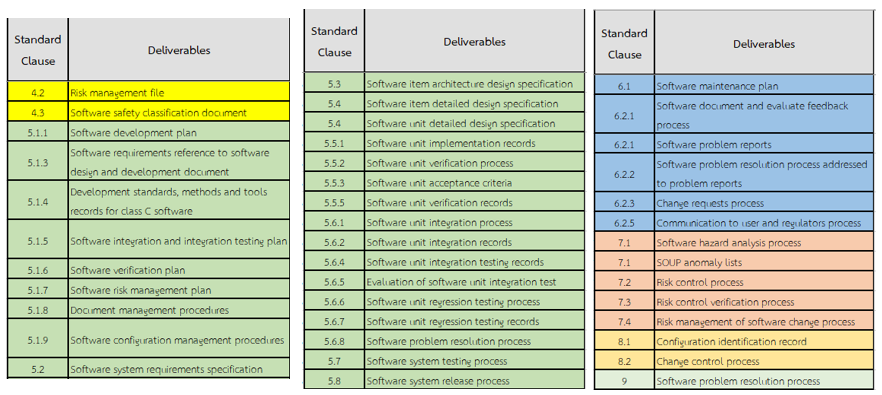
Figure 14 – Example Evidence List
Conclusion
This three-part article series has presented an overview of IEC 62304, the international standard for medical device software life cycle processes.
In the next article, we will continue exploring related standards — particularly IEC 60601-1 Clause 14, which governs safety requirements for programmable electrical medical systems (PEMS).
Stay tuned!
References
- Roger Pressman (2009). Software Engineering: A Practitioner’s Approach (7th ed.). McGraw-Hill, New York, USA.
- Ivan Mistrik et al. (2015). Software Quality Assurance: In Large Scale and Complex Software-Intensive Systems (1st ed.). Morgan Kaufmann, San Francisco, USA.
- Medical Device Software—Software Life Cycle Processes, ANSI/AAMI/IEC 62304, 2006.
Reference
[1] Roger Pressman. 2009. Software Engineering: A Practitioner’s Approach (7 ed.). McGraw-Hill, Inc., New York, NY, USA.
[2] Ivan Mistrik, Richard M. Soley, Nour Ali, John Grundy, and Bedir Tekinerdogan. 2015. Software Quality Assurance: In Large Scale and Complex Software-Intensive Systems (1st ed.). Morgan Kaufmann Publishers Inc., San Francisco, CA, USA.
[3] Medical Device Software—Software Life Cycle Processes, ANSI/AAMI/IEC 62304, 2006.
Author : Panita Meananeatra
ภาพปก : https://all-free-download.com/free-vector/download/medical-background-doctor-heart-cardiogram-decor_6829344.html